GKE Australia Indicators for Hydrogen Peroxide Processes
Hydrogen peroxide (H2O2) is not commercially available as a pure liquid, but only as an aqueous solution between 30-60%, as it can suddenly explode in pure form in contact with catalytic metal surfaces with release of oxygen (O2), water (H2O) and heat. H2O2 / H2O, mixtures are vaporized and used in the gas phase for sterilization of medical devices in healthcare or are used for room disinfection procedures. The reaction products oxygen (O2) and water (H2O) are non-toxic and are therefore used instead of toxic sterilization processes with EO or LTSF. Furthermore, these methods have the advantage to be carried out at low temperatures 20-60 ° C.
Room or isolator or incubator disinfection procedures
Room disinfection processes are performed by spraying / evaporating aqueous hydrogen peroxide solutions throughout the room or inside an incubator at room temperature. Before the humidity is reduced to below 10% RH. Thereafter, hydrogen peroxide mixtures of 30-50% are evaporated as an aerosol and sprayed into the room. In this case, the hydrogen peroxide gas concentration increases and until the desired concentration of e.g. 200-2,000 ppm is reached, the aerosol concentration is reduced and kept constant over time. During fumigation, it must be ensured that no water condenses on cold spots, otherwise hydrogen peroxide will dissolve in the condensate and disappears out of the gas phase. For this reason, the room including all equipment requires temperature stabilization beforehand in order to prevent condensation. If rooms are not rectangular, but designed in L- or T-shapes, or if machine installations obstruct natural convection, there is a risk that partial areas of the room may have insufficient H2O2 concentrations. In order to find out where the worst-case locations with low H2O2concentration are, biological indicators with G. stearothermophilus 10E6 are used on different carriers packaged in Tyvek envelopes. To validate the cleanroom disinfection process, 100-200 biological indicators (BI) are used. For routine monitoring, 20-30 BI are placed at the worst-case locations.
Room or isolator or incubator disinfection procedures
Room disinfection processes are performed by spraying / evaporating aqueous hydrogen peroxide solutions throughout the room or inside an incubator at room temperature. Before the humidity is reduced to below 10% RH. Thereafter, hydrogen peroxide mixtures of 30-50% are evaporated as an aerosol and sprayed into the room. In this case, the hydrogen peroxide gas concentration increases and until the desired concentration of e.g. 200-2,000 ppm is reached, the aerosol concentration is reduced and kept constant over time. During fumigation, it must be ensured that no water condenses on cold spots, otherwise hydrogen peroxide will dissolve in the condensate and disappears out of the gas phase. For this reason, the room including all equipment requires temperature stabilization beforehand in order to prevent condensation. If rooms are not rectangular, but designed in L- or T-shapes, or if machine installations obstruct natural convection, there is a risk that partial areas of the room may have insufficient H2O2 concentrations. In order to find out where the worst-case locations with low H2O2concentration are, biological indicators with G. stearothermophilus 10E6 are used on different carriers packaged in Tyvek envelopes. To validate the cleanroom disinfection process, 100-200 biological indicators (BI) are used. For routine monitoring, 20-30 BI are placed at the worst-case locations.
Hydrogen Peroxide Sterilization Processes
In the meantime there are more than 40 manufacturers producing H2O2 sterilizers on the world market with chambers of 20 – 200 liters. The sterilization takes place at temperatures of 30-60 °C. The material to be sterilized should also have the sterilization temperature before the addition of H2O2 in order to avoid condensation. After venting <1 mbar, H2O2-H2O mixtures (30 – 60% H2O2) are evaporated on hot plates into the chamber. With the intention of a better distribution of the gas mixture generated in the chamber air is added afterwards. However, it is an open question whether the existing H2O2 / H2O gas mixture mixes homogeneously with the air injected later on, which is very much related to the point of air addition and the type of loading. Air bubbles can be created that do not contain any H2O2 gas.
Since the H2O2v concentration in the gas phase is relatively low (2-10 mg/l), depletion of H2O2 on the material to be sterilized quickly occurs, when H2O2 is consumed and no sterilization can continue. Thereafter, remaining air, steam, H22O2, O2 mixtures are sucked off. Several cycles can be connected in series to obtain a better penetration and sterilization in the hollow devices. However, the penetration characteristics of these processes are much poorer compared to fractionated steam sterilization processes. For this reason, biological and/or chemical indicators must always be placed within the load having the most difficult penetration characteristics. Since BI cannot be placed inside packages (no evaluation before using the MP is possible), Process Challenge Devices (PCD) are used with biological or chemical indicators outside the load, which simulate the “worst-case“ load configuration. These PCDs must be validated with a worst-case load.
Hydrogen Peroxide Sterilization Processes
In the meantime there are more than 40 manufacturers producing H2O2 sterilizers on the world market with chambers of 20 – 200 liters. The sterilization takes place at temperatures of 30-60 °C. The material to be sterilized should also have the sterilization temperature before the addition of H2O2 in order to avoid condensation. After venting <1 mbar, H2O2-H2O mixtures (30 – 60% H2O2) are evaporated on hot plates into the chamber. With the intention of a better distribution of the gas mixture generated in the chamber air is added afterwards. However, it is an open question whether the existing H2O2 / H2O gas mixture mixes homogeneously with the air injected later on, which is very much related to the point of air addition and the type of loading. Air bubbles can be created that do not contain any H2O2 gas.
Since the H2O2v concentration in the gas phase is relatively low (2-10 mg/l), depletion of H2O2 on the material to be sterilized quickly occurs, when H2O2 is consumed and no sterilization can continue. Thereafter, remaining air, steam, H22O2, O2 mixtures are sucked off. Several cycles can be connected in series to obtain a better penetration and sterilization in the hollow devices. However, the penetration characteristics of these processes are much poorer compared to fractionated steam sterilization processes. For this reason, biological and/or chemical indicators must always be placed within the load having the most difficult penetration characteristics. Since BI cannot be placed inside packages (no evaluation before using the MP is possible), Process Challenge Devices (PCD) are used with biological or chemical indicators outside the load, which simulate the “worst-case“ load configuration. These PCDs must be validated with a worst-case load.
Advantages of H2O2 Processes
- The sterilizer does not require complex installation, an electrical connection is sufficient
- The process velocity is quicker than other low temperature sterilization processes
- At the end of the sterilization process H2O2 is decomposed in the non-toxic components water and oxygen
- No air removal cycles are necessary, just one deep vacuum is sufficient
- No toxic substances remain in the load, except small amounts of absorbed H2O2
- No special rooms with flow-through aeration are required in comparison using EO sterilization processes
Disadvantages of H2O2 Processes
Chemical Reaction kinetics
H2O2 is a very good oxidant and decomposes without stabilization explosively into water (H2O) and oxygen (O2):

H2O2is not available in pure solution and requires H2O for stabilization and transportation. Typically it is available in aqueous solutions, e.g. in cartridges with the maximal allowed concentration based on safety precaution: H2O2 : H2O ~ 60 : 40

H2O2 solutions decompose over time, also the diffusion of H2O2 through the plastic cartridges is possible. Chemical and biological indicators should not be stored together with H2O2 cartridges or bottles, since they react with the H2O2-vapour over time!

The free radicals inactivate the germs very fast, however only small amounts are present which also recombine again. Water stabilizes the H2O2 molecule by solvation and therefore decreases the concentration of the free radicals, which are responsible for sterilization.

The NX-versions of the Sterrad series concentrate the H2O2 by vaporization. Water evaporates faster than H2O2 (100°C, 100 kPa) and concentrates the H2O2 (150°C, 100 kPa) concentration to over 90%.Alternatively to H2O2 peracetic acid can be used.
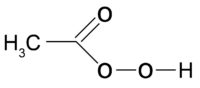
Peracetic acid also splits off a•OH-radical. Also the sodium salt of the peracetic acid is used in liquid solutions in the Steris-1-process for high-level disinfection.Also there are processes which use peracetic acid and H2O2 in the gas phase.
Physical relationships in describing the penetration of Hydrogen Peroxide in hollow devices
Introduction
VHPO (vaporized hydrogen peroxide oxide) / plasma sterilization processes are increasingly being used in the healthcare sector as low-temperature sterilization processes and replace ethylene oxide sterilization processes. Their problem is that residual EO absorbed in plastic and on plastic surfaces takes longer to desorb and thereby instruments being sterilized in EO sterilization processes are not available for a long time after sterilization, because they require a long quarantine time for EO desorption.
It is known that VHPO sterilization processes have limited penetration characteristics of the sterilizing agent in hollow devices and tubes compared to steam sterilization processes. Therefore, the efficiency of the processes are very different depending on the processes used.
Physical basic information
1. VHPO Concentration inside the sterilization chamber:
H2O2 dissolved in water is the basis for the production of the VHPO gas in the sterilizer chamber in all processes. Concentrations of 35-60% VHPO / water are used. In some processes, the solution is concentrated prior to evaporation, as water evaporates much faster (H2O boiling point 100 °C and H2O2 boiling point 150 °C under normal conditions) and thus the concentration of VHPO can be increased up to 90% (Sterrad NX). However, the concentration of the starting liquid phase is only secondary to the concentration of H2O2 in the gas phase. It depends on the amount of liquid injected, the concentration of the solution and the volume of the sterilizer chamber.
Sterilization methods with H2O2 are usually low pressure methods. Almost all known sterilization processes initially use a vacuum < 1 mbar and inject the liquid mixture into the chamber, which then evaporates immediately. This is followed by a holding time where no further VHPO is replenished. Essentially, a VHPO and steam mixture is formed. VHPO is very reactive and there are several chemical approaches to inactivate germs. The reaction consists of the oxidation of proteins to form oxidation products, steam and oxygen O2. As a result of these reactions, VHPO is consumed and quickly lowers the relatively VHPO concentrations of 2-10 mg / l, since it can react with all objects in the sterilizer (chamber wall, packaging material, instruments).
VHPO is stabilized with H2O in the liquid phase. However, to which extent it plays a role in the inactivation of germs in the gas phase is currently still under discussion, especially as the VHPO concentration is decreasing and the water concentration is increasing during the reaction in all those processes.


Biological Indicators
In the submerged fermentation process, gke produces large batches of biological indicators with homogeneous resistance. Conventional “solid state” fermentation processes on petri dishes where the spores are scraped off and indicate inhomogeneous resistance values. These generate non-linear semilogarithmic survival curves. The semilogarithmic survival curves of the gke BIs are linear.
By using the fermenter method, gke is able to adjust the resistances of the BI in a certain range according to the requirements of the customers and has batch sizes in the range of 10E10 – 10E12 CFUs. For monitoring of H2O2 processes gke produces purified spore suspensions which are applied to the carriers in a reproducible monolayer.
All gke biological indicators comply with the standard EN ISO 11138 series, European and American Pharmacopeia (EP + USP). The specifications for population and D-value are documented in the certificate for each batch included in every package.
The biological indicators (spore discs, strips and SCBIs) contain G. Stearothermophilus spores, that are immobilized on different carriers (e.g. glass fiber, Tyvek, stainless steel, PET). For evaluation a microbiological laboratory is necessary. Growth medium tubes for incubation of biological indicators are also available at gke. gke self-contained biological indicators already contain a growth medium and don‘t need a microbiological lab for evaluation.
Biological Indicators
1. gke Steri-Record® Self-contained biological indicators
Mini-Bio-Plus self-contained biological indicator (SCBI) uses a plastic vial containing a spore plate and glass ampoule with a growth medium and pH-indicator inside. It is used for validation and routine monitoring of hydrogen peroxide sterilization processes without using a microbiological laboratory.
The SCBI are available with different carrier materials.
They can also be used inside gke process challenge devices (Bio-PCDs), All SCBI fulfil the requirements according to EN ISO 11138-1.
1.1. Standard SCBIs
G. Stearothermophilus available with population 106 CFU/SCBI

| Art.-No. | Quantity | Product Code | Carrier | Colour of cap |
|---|---|---|---|---|
| 327-601 | 10 | B-V-G-MBP-10-6 | Glass fiber | Light Grey |
| 327 -605 | 50 | |||
| 327-610 | 100 | |||
| 337-601 | 10 | B-V-T-MBP-10-6 | Tyvek | Colourless |
| 337-605 | 50 | |||
| 347-601 | 10 | B-V-ST-MBP-10-6 | Stainless steel | Dark Grey |
| 347-605 | 50 | |||
| 357-601/td> | 10 | B-V-P-MBP-10-6 | PET | Light Green |
| 357-605 | 50 |
1.2. Instant-SCBIs for immediate release
G. Stearothermophilus available with population 106 CFU/SCBI
The Instant-Mini-Bio-Plus SCBI contains a type 4 chemical indicator allowing that the result of hydrogen peroxide sterilization processes can be instantly evaluated at the end of the process. Therefore, it is not necessary to wait for the result of the SCBI incubation since the type 4 indicator provides equivalent or better information about the result of the sterilization process according to EN ISO 11140-1
| Art.-No. | Quantity | Product Code | Carrier | Colour of cap |
|---|---|---|---|---|
| 327-651 | 10 | B-V-G-I-MBP-10-6 | Glass fiber | N/A |
| 327 -655 | 50 | |||
| 327-650 | 100 |

2. gke Steri-Record® Incubators and accessories
The incubation temperature is visible in the display. All incubators are either available with an aluminium block to incubate SCBIs or alternatively without aluminium block. In this case an aluminium block available for different applications has to be ordered separately. The plug contains a CE conformity for the low voltage directive.
2.1. Dry Bath Incubators

| Art.-No. | Product Code | Temperature | Application |
|---|---|---|---|
| With aluminium block for SCBIs | |||
| 610-120 | I-57-AB-MBP | 57 | to incubate G. stearothermophilus biological indicators |
| 6610-121 | I-V-AB-MBP | 30 – 60 | variable temperature selection |
| 610-122 | I-V-T-AB-MBP | variable temperature selection and programming of the incubation time | |
| Without aluminium block | |||
| 610-110 | I-57 | 57 | to incubate G. stearothermophilus biological indicators |
| 610-111 | I-V | 30 – 60 | variable temperature selection |
| 610-112 | I-V-T | variable temperature selection and programming of the incubation time | |
2.2. Accessories
Aluminium blocks to insert SCBIs (12 pcs each).
| Art.-No. | Product Code | Diameter | Application |
|---|---|---|---|
| 610-113 | I-AB-MBP | 10 mm | for all gke Steri-Record® Mini-Bio-Plus SCBIs |

2.3 Crusher for SCBIs
To activate all gke SCBIs. The gke incubator already includes a crusher.

| Art.-No. | Product Code | Material | Application |
|---|---|---|---|
| 224-002 | I-C | Stainless steel | 1 |
| 224-004 | I-PC | Plastic | 10 |
3. Spore Suspensions
All spore suspensions are delivered in 10 ml glass bottles with a septum, suspended in 40% ethanol/water and comply with EN ISO 11138-1.
| Art.-No. | Product Code | Population[CFU/ml] | Population per bottle | Characteristics |
|---|---|---|---|---|
| 228-107 | B-S-F-V-SUS-10-7 | 107 | 108 | purified |
| 228-108 | B-S-F-V-SUS-10-8 | 108 | 109 | |
| 228-997 | Determination H2O2 | |||

4. gke Steri-Record® Growth medium
Test tubes (diameter: 16,1 mm) with CASO-Boullion (TSB) with pH-indicator and aluminium screw cap with seal. The test tubes have optimized dimensions and volume to fit all kind of spore strips. If germs are growing the pH-indicator changes its colour and allows a quick evaluation of the result

| Art.-No. | Quantity | Product Code | Process | Germ |
|---|---|---|---|---|
| 223-010 | 10 | B-S-V-CM | steam, hydrogen peroxide | G. stearo-thermophilus |
| 223-100 | 100 |
Chemical Indicators
5. Batch and Process Monitoring
The chemical indicators only provide the information about the quality of the sterilization and disinfection process with hydrogen peroxide on the location where the indicator is placed.
Several Process Challenge Devices (PCD) can be selected to monitor the process. gke will help you to select the appropriate PCD for routine monitoring.
After sterilization the self-adhesive indicator can be used for documentation.
The gke chemical indicators comply with EN ISO 11140-1.
All indicators and labels for hydrogen peroxide sterilization processes have a plastilc foil carrier since paper must not be used in those processes.
5.1. Process Challenge Devices and Testsets
The H2O2 Batch Monitoring System (BMS) ensures that the hydrogen peroxide gas penetrates into the most-difficult areas inside the load or all locations in the isolator. The air removal and penetration characteristics of hydrogen peroxide are very different depending on the sterilizer model and program and very much dependent on the material to be sterilized. Therefore, gke does not offer a fixed combination of indicator and PCD, the selection of the PCD depends on the performance of the hydrogen peroxide process on the one hand and the requirements of the load and material properties on the other hand. It must be ensured that the selected PCD simulates the load to be sterilized. After selecting the appropriate PCD, it can be ordered separately and used with biological or chemical indicator strips.
5.1.1. Helix-Testset and PCDs
| Art.-No. | Product Code | tube | Quantity | |
|---|---|---|---|---|
| 200-016 | PM-HPCD-TS-10 | Testset with 10 PCDs | 10 | |
| 200-525 | PM-HPCD-5-25 | 5 | 25 | 1 |
| 200-550 | PM-HPCD-5-50 | 5 | 50 | |
| 200-575 | PM-HPCD-5-75 | 5 | 75 | |
| 200-510 | PM-HPCD-5-100 | 5 | 100 | |
| 200-425 | PM-HPCD-4-25 | 4 | 25 | |
| 200-450 | PM-HPCD-4-50 | 4 | 50 | |
| 200-475 | PM-HPCD-4-75 | 4 | 75 | |
| 200-325 | PM-HPCD-3-25 | 3 | 25 | |
| 200-350 | PM-HPCD-3-50 | 3 | 50 | |
| 200-025 | PM-HPCD-2-25 | 2 | 25 | |

5.1.2 Compact-PCD-Testset and Compact-PCDs, grey
The testsets contain PCDs with different penetration characteristics. All PCDs can be used with gke biological or chemical indicators
5.2. Self-adhesive Indicator Strips for Process Monitoring
To be used in all gke BMS/PMS process challenge devices. EN ISO 11140-1 Type 2 Indicator in combination with a PCD.
Indicators for type 2 indicator systems
| Sensitivity level | Art. No. | Indicator Starting Colour | Indicator Pass Colour |
|---|---|---|---|
| 1 | 214-201 |
6. Self-adhesive Package Monitoring Indicators
The indicators according to EN ISO 11140-1 Type 4 are placed into packages or containers to monitor the relevant variables of sterilization processes. Package monitoring indicators only provide sterility information at the position inside the chamber where they are located. Alternatively those indicators can also be used to monitor disinfection processes (e.g. in rooms or isolators). After sterilization they can be adhered for documentation
The indicators are available on card with 16 self-adhesive indicators with different specifications, dose = 2.3 mg/l H2O2 , 50 °C, alternative: 2 min, 4 min, or 6 min. The indicator can also be used for longer sterilization times, higher temperatures and concentrations.
Type 4 indicators
| Sensitivity level | Art. No. | Indicator Starting Colour | Indicator Pass Colour |
|---|---|---|---|
| 1 | 214-251/-253 |  |
 |
7. Labels with process indicators to be used in printers
The labels with process indicator according to EN ISO 11140-1 type 1 are used in printers with and without cutting device. After the information is printed the label can be adhered on sterilization packs or pouches and after opening them for medical treatment they can be transferred to the patient documentation. The chemical indicator provides logistic information if the package has passed a sterilization process.
7.1. Endless label with 7 cm width to be used in printers with cutting device
| Art.-No. | Product Code | Length | Outside roll diameter | Roll core |
|---|---|---|---|---|
| 214-390 | C-V-L-1-70-DA | 60 m | 14,7 cm | 3 |

"*" indicates required fields


